

paper) Subjects: LCSH: Chemistry-Study and teaching-Congresses. | Description based on print version record and CIP data provided by publisher resource not viewed. | Includes bibliographical references and index.
Chemdoodle delocalized benzene series#
Description: Washington, DC : American Chemical Society, | Series: ACS symposium series 1312 | Based on the 254th American Chemical Society national meeting, held in 2017, in Washington, DC. Reeves, editor (Department of Chemistry, Tuskegee University, Tuskegee, Alabama, United States) sponsored by the ACS Division of Chemical Education. Meeting (254th : 2017 : Washington, D.C.) Title: Using computational methods to teach chemical principles / Alexander Grushow, editor (Department of Chemistry, Biochemistry, and Physics, Rider University, Lawrenceville, New Jersey, United States), Melissa S.
Library of Congress Cataloging-in-Publication Data Names: Grushow, Alexander, editor. Sponsored by the ACS Division of Chemical EducationĪmerican Chemical Society, Washington, DC
Reeves, Editor Department of Chemistry Tuskegee University Tuskegee, Alabama, United States Using Computational Methods To Teach Chemical Principles Alexander Grushow, Editor Department of Chemistry, Biochemistry, and Physics Rider University Lawrenceville, New Jersey, United States Using Computational Methods To Teach Chemical Principles
Chemdoodle delocalized benzene software#
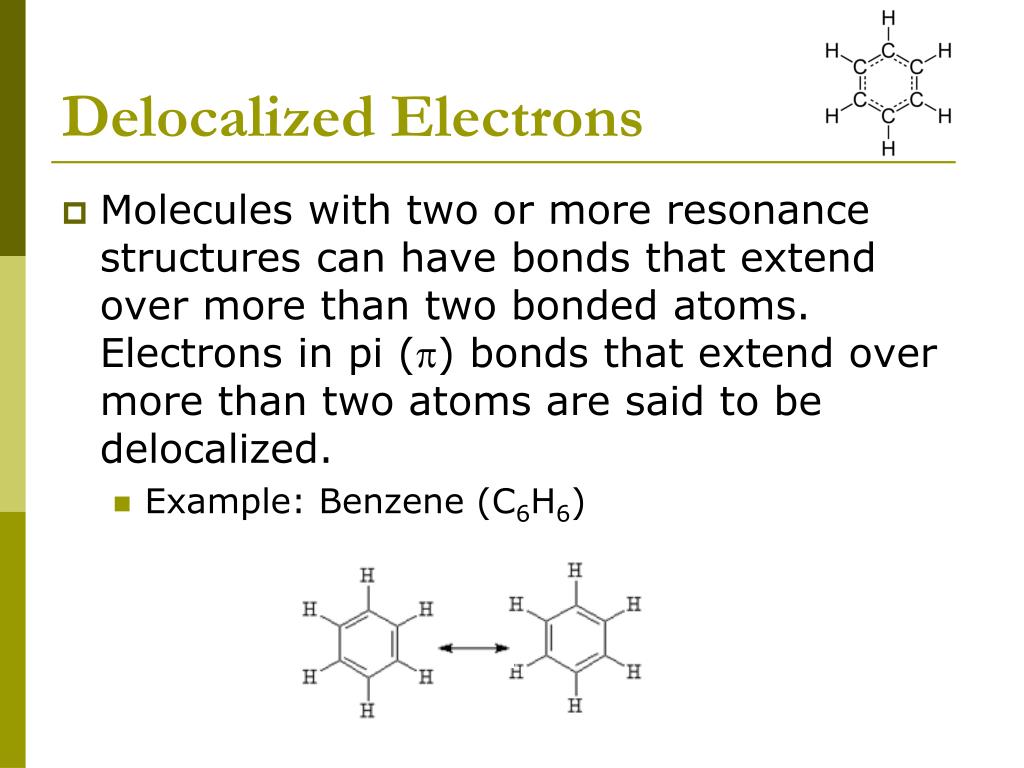
Ħ Process Oriented Guided Inquiry Learning Computational Chemistry Experiments: Revisions and Extensions Based on Lessons Learned from Implementation.ħ Chem Compute Science Gateway: An Online Computational Chemistry Tool.Ĩ Using Computational Chemistry to Extend the Acetylene Rovibrational Spectrum to C2T2.ĩ Introducing Quantum Calculations into the Physical Chemistry Laboratory.ġ0 Learning by Computing: A First Year Honors Chemistry Curriculum.ġ1 Integrating Computational Chemistry into an Organic Chemistry Laboratory Curriculum Using WebMO.ġ2 Computational Narrative Activities: Combining Computing,Context, and Communication To Teach Chemical Concepts.ġ3 Computational Chemistry as a Course for Students Majoring in the Sciences.ġ4 Beyond the Analytical Solution: Using Mathematical Software To Enhance Understanding of Physical Chemistry.ġ5 A Lab Course in Computational Chemistry Is Not About Computers.ġ6 Discovery-Based Computational Activities in theUndergraduate Chemistry Curriculum.ġ7 Using the Hydrogen Bond as a Platform for the Enhancement of Integrative Learning. These six protons are equivalent because they have the same electronic environment.1 Using Computational Methods To Teach Chemical Principles: Overview.Ģ Molecular Dynamics Simulations in First-Semester General Chemistry: Visualizing Gas Particle Motion and Making Connections to Mathematical Gas Law Relationships.ģ Using Electronic Structure Calculations To Investigate the Kinetics of Gas-Phase Ammonia Synthesis.Ĥ Modeling Reaction Energies and Exploring Noble Gas Chemistry in the Physical Chemistry Laboratory.ĥ How Can You Measure a Reaction Enthalpy without Going into the Lab?. It reflects into the strong singlet NMR signal of the six equivalent protons ( Hydrogen). Hence, electron density flows uniformly around each carbon and hydrogen atom in benzene. This gain in stability occurs as the six p-electrons of the carbon atoms are delocalized above and below the ring forming a continuous pi-bond instead of being localized/fixed between two carbon atoms. The resonance hybrid has energy below the energy of the Kekule structures and is more stable. This resonance in the benzene has been confirmed by electron diffraction, X-ray diffraction, and molecular spectroscopy. However, its inability to saturate suggests that the actual structure of benzene does not correspond to any one of the above individual structures but rather to a combination of them (i.e., Resonance hybrid). Each above structure having conjugated double bonds should easily undergo addition (e.g., hydrogenation/saturation) reactions.


 0 kommentar(er)
0 kommentar(er)
